L3, M1 and M2 internships
Wavelength-selective photocleavable protecting groups for the synthesis and activation of biologically relevant peptides
Small peptides are attractive and underexploited biomolecules, that occupy an intermediate molecular space between that of traditional drug-like compounds and much larger “biologics”. Because their sequence can be directly derived from proteins, they can closely reproduce specific side-chains arrangement and should thus incarnate the simplest protein mimetics. However, out of their proteic context, peptides are usually highly flexible and poorly stable in biological media. To overcome these limitations, intense research efforts have been devoted to the development of cyclopeptides, in which late-stage side-chains modification remains nevertheless challenging.
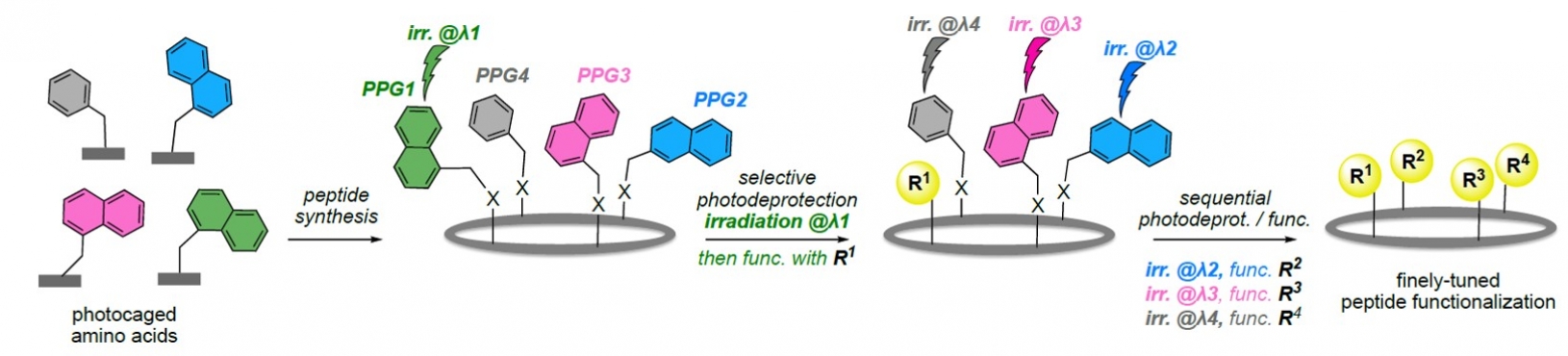
The aim of this research project is to synthesize new photocaged amino acids building blocks (i.e. whose reactivity can be selectively modified under the effect of light irradiation) and incorporate them into cyclopeptides of biological interest. By adjusting the photophysical properties of the photocleavable protecting groups (PPGs), fine tuning of side-chain reactivity could be reached. This opens new perspectives for both the development of novel synthetic routes to biologically relevant peptide drugs showing increased stability and the activation of biological processes in living systems with a high degree of spatiotemporal control.This interface project centered on organic synthesis is part of an on-going collaboration with external partners Dr. Roba Moumné (CPCV, Sorbonne Université, peptide chemistry) and Dr. Eric Deprez (LBPA, ENS Paris-Saclay, cell imaging and applications of photoactivatable probes).
In this context, the recruited intern will be in charge of the synthesis and characterization of several photo-sensitive compounds, taking advantage of its theoretical and practice-based knowledge in the field of organic chemistry. A training in photophysics and photochemistry (study of the properties and reactivity of the synthesized products) will be provided.
Short stays in partners laboratories could also be organized depending on applicant background, interest and progress of the project.
For more information contact Dr Nicoals Bogliotti.
References on related work:
PPSM: Visible Light Initiated Palladium-catalyzed Cross-couplings by PPh3 Uncaging from Azobenzene Ruthenium-Arene Complex. L. Rocard, J. Hannedouche, N. Bogliotti, Chem. Eur. J. 2022, 28, e202200519.